Synthesis and thermal analysis of 2,4,6-trinitroresorcinol
960 viewsDOI:
https://doi.org/10.54939/1859-1043.j.mst.78.2022.86-92Keywords:
2,4,6-trinitroresorcinol; Styphnic acid; Thermal decomposition.Abstract
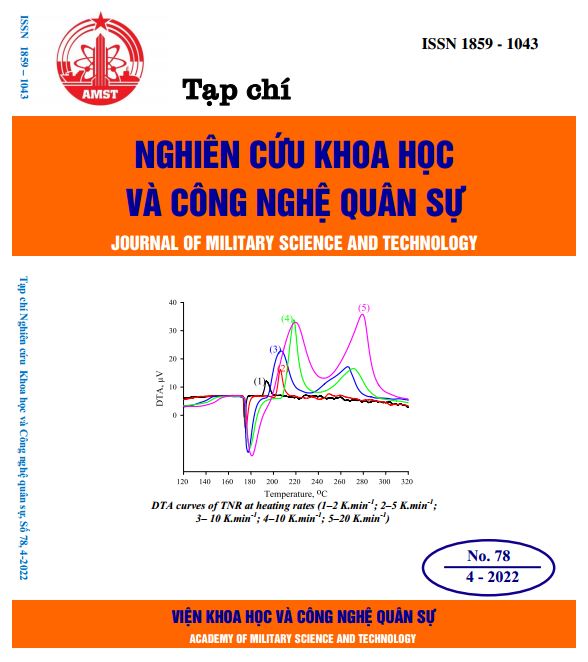
In this work, the preparation of 2,4,6-trinitroresorcinol by the sulfonation-nitration method of resorcinol through three stages was investigated. The results showed that the 2,4,6-trinitroresorcinol synthesis efficiency reached the highest value (82-83%) when the molar ratio of HNO3/resorcinol was 3.45/1, the temperature of the nitrate stage was 30-70 ºC, and the concentration of HNO3 solution was 72%. Besides, the results of 2,4,6-trinitroresorcinol thermal analysis by TGA/DTA methods demonstrated that there are one melting point and two thermal decomposition points in the temperature range from 50 to 400 ºC.
References
[1]. K. G. Balachandar, A. Thangamani, “Design of new energetic materials based on derivatives of 1,3,5-trinitrobenzenes: A theoretical and computational prediction of detonation properties, blast impulse and combustion parameters”, Heliyon, Vol. 6, I. 1, (2020), e03163, pp 1-20.
[2]. P. Dhamodharan, K. Sathya and M. Dhandapani, “Studies on synthesis, structural, luminescent and thermal properties of a new non-linear optical crystal:4-amino-4H-1,2,4-triazol-1-ium-3-hydroxy-2,4,6-trinitrophenolate”, Physica B: Condensed Matter, Vol. 508, (2017), pp. 33-40.
[3]. B. A. Bydal, “2,4,6-trinitroresorcinol", Org. Prep. Proced. Int., Vol. 5, No 6 (1973), pp 271-273.
[4]. T. Kametani, K. Ogasawara, “An improved synthesis of styphnic acid”, Chem. Pharm. Bull. Vol. 15, No. 6 (1976), 893.
[5]. D. A. Salter, R. J. J. Simkins, "A new process for the manufacture of styphnic acid”, report No. 8/R/69, (1969).
[6]. T. Urbanski, “Chemistry and Technology of Explosives”, Pergamon press, Oxford-London, Vol. I, (1964), pp 538-541.
[7]. Sh. Weng, W. Wu, Z. Guo, F. Meng, Y. Chen, W. Chen, “The formation mechanism and thermal decomposition kinetics of 2,4,6-trinitroresorcinol in the dinitrobenzene production”, Process Saf. Environ. Protect., Vol. 157 (2022), pp 167-174.
[8]. M. Xue, D. Zh. Huang, K. Yang, L. Chen, Zh. Zheng, Y. Xiang, Q. Huang, J. Wang, “Measurement, correlation of solubility and thermodynamic properties analysis of 2,4,6-trinitroresorcinol hydrate in pure and binary solvents”, J. of Mol. Liquids, Vol. 330, (2021), 115639, pp 1-14.